Comprehensive Age-related Macular Degeneration Market Analysis: Trends and Developments Across All Types
According to DelveInsight’s analysis, the market for age-related macular degeneration is anticipated to increase during the forecast period (2025–2034), owing to the rise in the launch of emerging therapies and healthcare spending globally.
New York, USA, June 16, 2025 (GLOBE NEWSWIRE) -- Comprehensive Age-related Macular Degeneration Market Analysis: Trends and Developments Across All Types
According to DelveInsight’s analysis, the market for age-related macular degeneration is anticipated to increase during the forecast period (2025–2034), owing to the rise in the launch of emerging therapies and healthcare spending globally.
Age-related macular degeneration (AMD) is a major cause of permanent blindness globally, severely impacting patients' lives. As people live longer, AMD cases are increasing, placing a heavy financial strain on healthcare systems due to the costly treatments currently available. In 2021, roughly 1.3% of the population had wet AMD annually.
The number of AMD cases is expected to increase significantly by 2034 because people are living longer and are more likely to develop AMD as they age. This poses a significant public health problem because the disease affects central vision, lowers quality of life, and requires more healthcare resources for treatment and care.
While there is no cure, early diagnosis and treatment can slow its progression. Wet AMD is managed with anti-VEGF injections, while dry AMD management involves lifestyle adjustments like diet, supplements, quitting smoking, and regular check-ups.
Discover more about the AMD market in detail @ Age-related Macular Degeneration Market Report
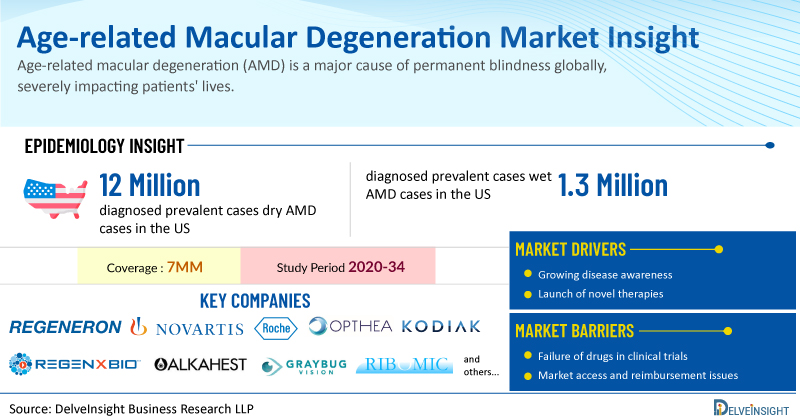
DelveInsight has recently released epidemiology-based market reports focusing on AMD, including Dry AMD and Wet AMD. These reports include a comprehensive understanding of current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Additionally, the reports feature an examination of prominent companies working with their lead candidates in different stages of clinical development. Let’s deep dive into the assessment of these different types of AMD markets individually.
Dry Age-related Macular Degeneration Market
Dry age-related macular degeneration is a common, progressive eye condition affecting the macula, the part of the retina responsible for central, detailed vision. While it typically progresses slowly and doesn't cause complete blindness, it can lead to vision loss. In 2023, the US had the highest number of dry AMD cases among the 7MM, with approximately 21 million cases, and this number is projected to increase.
Individuals with dry AMD usually experience impaired central vision but maintain peripheral vision, allowing them to continue many daily activities. Although there is currently no cure, treatments aim to slow disease progression. Supplements containing vitamins C and E, lutein, zeaxanthin, zinc, and copper may help slow the advancement of dry AMD.
SYFOVRE and IZERVAY were approved by the FDA in 2023 to slow the progression of geographic atrophy, a late-stage form of dry AMD. These injectable medications can slow disease progression but cannot reverse existing damage or restore lost vision. Both SYFOVRE by Apellis and IZERVAY by Astellas are now competing in the geographic atrophy treatment market. Comparing the efficacy of IZERVAY and SYFOVRE is challenging due to differences in study designs. Post-marketing safety concerns pose a significant threat to both drugs.
Several companies with their lead products, such as Alkeus Pharmaceuticals (ALK-001), Allegro Ophthalmics (Risuteganib), Stealth BioTherapeutics (Elamipretide), Lineage Cell Therapeutics (OpRegen), and others are working on developing a new treatment for dry AMD.
As per DelveInsight analysis, the US dry AMD market was valued at approximately USD 800 million in 2023 and is expected to grow between 2025 and 2034. According to DelveInsight’s estimation, the overall market size in the United States for dry AMD is anticipated to increase, and this will further contribute to the overall market size during the forecast period (2025–2034).
Dive deeper for rich insights into the Dry AMD Market Trends and Analysis
Wet Age-related Macular Degeneration Market
Wet age-related macular degeneration is a form of AMD characterized by rapid central vision loss. This occurs when abnormal blood vessels develop in the eye, leaking fluid and blood that damages the macula. While wet AMD, also known as neovascular AMD, only accounts for about 10% of all AMD cases, it is responsible for approximately 90% of the vision loss associated with AMD. In 2024, the US had over 1.3 million wet AMD cases, and these numbers are projected to increase significantly by 2034.
Fifteen years ago, photodynamic therapy was the main treatment. However, in the early 2000s, anti-VEGF drugs such as ranibizumab (LUCENTIS), aflibercept (EYLEA), brolucizumab (BEOVU), faricimab (VABYSMO), and ranibizumab (SUSVIMO) became available. These drugs are injected directly into the eye to inhibit the formation of new blood vessels and reduce leakage from existing abnormal vessels. Some patients have regained lost vision using these treatments. Low vision aids can also help improve clarity.
The first anti-VEGF drug used was bevacizumab (AVASTIN), initially approved for colon cancer in 2004 and later used off-label for wet AMD. Despite newer drugs, bevacizumab still holds a significant portion (50%) of the US wet AMD market. Pegaptanib (Macugen) was approved specifically for wet AMD in December 2004, but its marketing has been discontinued.
However, there are safety and potency concerns regarding off-label bevacizumab, particularly due to variations in compounding pharmacies' repackaging processes. An on-label version could address these issues. The patent for LUCENTIS expired in 2020, leading to market decline due to biosimilar competition. EYLEA's patent expired in 2023, also impacting its sales. The COVID-19 pandemic further affected the market in 2020.
SUSVIMO, a ranibizumab injection delivered via an implant, was approved in 2021, potentially to recover market share lost by LUCENTIS. Brolucizumab (BEOVU) and Faricimab (VABYSMO), approved in 2020 and 2022, respectively, offer the advantage of less frequent injections.
The unmet needs of treatment warrant the development of new therapies. Research is underway to identify treatment approaches that may address existing treatment challenges, including regimen, mode of delivery, and nonresponse.
Key players include Kodiak Sciences (KSI-301), Opthea Limited (OPT-302), Alkahest, Inc. (AKST4290), Ribomic USA (RBM-007), Regenxbio (RGX-314), and Outlook Therapeutics (ONS-5010). These drugs are expected to reach the market in the forecast period (2025-2034).
Other companies like EyePoint Pharmaceuticals (EYP-1901), 4D Molecular Therapeutics (4D-150), PanOptica (PAN-90806), Clearside Biomedical (CLS-AX), AsclepiX Therapeutics (AXT 107), and others are also involved in developing therapies for the treatment of wet age-related macular degeneration.
As per DelveInsight analysis, the wet AMD market across seven major markets was valued at approximately USD 10 billion in 2024 and is projected to grow by 2034, driven by new therapies and increasing prevalence.
For a comprehensive view of the wet AMD market, check out the Wet AMD Market Assessment
Related Ophthalmology Reports
Geographic Atrophy Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key geographic atrophy companies, including Apellis Pharmaceuticals, Iveric Bio (formerly Ophthotech Corporation), Alkeus Pharmaceuticals, Hemera Biosciences, Allegro Ophthalmics, Stealth BioTherapeutics, Regenerative Patch Technologies, Novartis, Roche, Ionis Pharmaceuticals, CellCure Neurosciences (a subsidiary of Lineage Cell Therapeutics), among others.
Intermediate AMD Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key intermediate AMD companies, including Novartis, Allegro Ophthalmics, among others.
Diabetic Macular Edema Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key DME companies, including Adverum Biotechnologies, Graybug Vision, Roche, Novartis, KalVista Pharmaceuticals, Ocuphire Pharma, Oxurion, YD Life Science, Novartis, Allergan (AbbVie), Molecular Partners, Allergo Ophthalmics, Bausch Health, Kodiak Sciences, Clearside Biomedical, among others.
Myopic Macular Degeneration Market
Myopic Macular Degeneration Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key myopic macular degeneration companies, including Roche, Bayer, Regeneron Pharmaceuticals, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
